Learn about this topic in these articles:
- Boron-11 The atomic mass of boron is 10.81 u. And 10.81 u is a lot closer to 11u than it is to 10u, so there must be more of boron-11. To convince you fully, we can also do a simple calculation to find the exact proportion of boron-11 using the following formula: ((10 u)(x)+(11 u)(1-x))/(100%)=10.81u Where u is the unit for atomic mass and x is the proportion of boron-10 out of the total boron.
- We prepared four sets of cBN crystals combining natural nitrogen (99.6% 14 N and 0.4% 15 N) with different boron isotope compositions, including nat B (21.7% 10 B and 78.3% 11 B), enriched (99.3%).
- Boron stable-isotope ratios and concentrations of boron in 17 ground-water samples and tritium concentrations in 9 ground-water samples collected in 2004 were used to identify geochemical differences between potential sources of boron in ground water near Beverly Shores, Indiana.
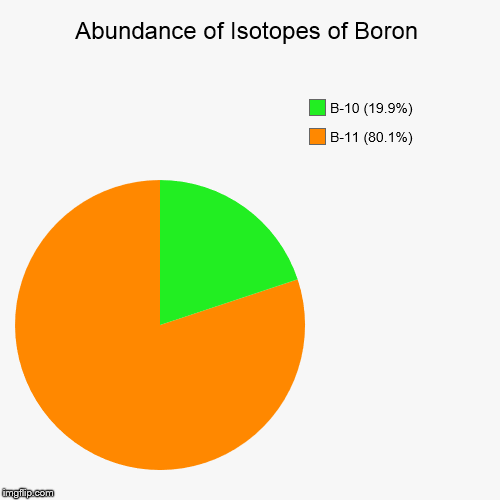
Calculator time! 19.9% of all Boron atoms are Boron-10. 80.1% are Boron-11. Calculate the approximated relative atomic mass of Boron, to one decimal place.
detection of slow neutrons
- In radiation measurement: Slow-neutron detectors
In the lithium-6 (6Li) and boron-10 (10B) reactions, the isotopes of interest are present only in limited percentage in the naturally occurring element. To enhance the conversion efficiency of lithium or boron, samples that are enriched in the desired isotope are often used in the fabrication of detectors. Helium-3 (3He)…
Read More
Boron Isotopes Model
properties of boron
Boron Isotopes Ph
Boron Isotopes Abundance
- In boron: Properties, occurrence, and uses
…mixture of two stable isotopes—boron-10 (19.9 percent) and boron-11 (80.1 percent); slight variations in this proportion produce a range of ±0.003 in the atomic weight. Both nuclei possess nuclear spin (rotation of the atomic nuclei); that of boron-10 has a value of 3 and that of boron-11, 3/2, the…
Read More
